The discharge of impulses in motor nerve fibres.
Edgar D Adrian
Detlev W. Bronk
Part 1. Impulses in single fibres of the phrenic nerve.
J Physiol. Sep 18; 66(1):81-101, 1928 and
Part II. The frequency of discharge in reflex and voluntary contractions.
J. Physiol. Mar 20; 67(2): 119-151, 1929.
The Analog Language of the Motoneuron
The action potential is binary, in the sense that it either occurs at full size or not at all, but that does not make the brain a binary logic circuit. The action potential might even be incidental - just an implementation detail, or even a design liability that must be overcome. This view was championed by Edgar Adrian, co-discoverer of the all-or-nothing nature of the action potential. He and Detlev Bronk published two landmark papers in 1928 and 1929 arguing that rhythmic motoneuron spike trains command muscle contraction in a continuous analog way, on the basis of firing rate.
They were not Adrian's first single axon recording studies. In 1926 he published a series of papers describing very similar firing patterns in sensory axons. As usual for sensory neurons, the stimulus features correlated with firing rate changes were easily determined, but the way they were interpreted by the brain was not. The key to the motoneuron papers was access to the principle applied by the muscle to transform the spike-coded signal to movement.
Adrian and Bronk
Edgar Douglas Adrian was one of the fathers of modern neurophysiology. He was born in London in 1889, and received his B.A. degree in 1911 from Trinity College, Cambridge. He stayed at Cambridge and studied with Keith Lucas. Adrian and Lucas are credited with discovery of the "all-or-nothing law" of action potentials. Adrian became a Fellow of Trinity College in 1913. He received a medical degree from St. Bartholomew's Hospital and worked there throughout World War I. Lucas was killed in an aviation training accident during the war, and in 1919 Adrian returned to Cambridge to head up the Lucas laboratory. He became a Fellow of the Royal Society in 1923, Foulerton Professor of the Royal Society in 1929, and Professor of Physiology at Cambridge in 1937. He received the Nobel Prize in Physiology and Medicine in 1932, sharing the prize with Sir Charles Sherrington. He was President of the Royal Society from 1950-1955, and of the Royal Society of Medicine from 1960-1962. He was President of the British Association for the Advancement of Science, and was made Baron of Cambridge in 1955. Among physiologists he is usually referred to as Lord Adrian.
Detlev Wulf Bronk was born in 1897 in New York, and received his B.A. in electrical engineering from Swarthmore College in 1920. During World War I he underwent training as a naval aviator and was commissioned an ensign in the Naval Air Corps. He completed a M.S in physics but in 1924 shifted his research to Physiology and received a PhD in physics and physiology from Michigan in 1927. In 1928 he was awarded a National Research Council fellowship for postdoctoral studies with Adrian at Cambridge. The National Research Council turned down his application for the second year of his fellowship, and he returned to teach at Swarthmore College. He obtained a research appointment as Johnson Professor of Biophysics and Director of the Eldridge Reeves Johnson Foundation for Medical Physics at the nearby University of Pennsylvania. He maintained this productive arrangement for teaching and research for many years. He is remembered as one of the architects of national research policies in the US in the second half of the 20th century. During World War II he became a member of the National Research Council, and ultimately became its chairman. He became president of Johns Hopkins University 1949, and president of the National Academy of Sciences in 1950. He was an influential advocate for the creation of the National Science Foundation and was Chairman of the National Science Board. He was president of The Rockefeller University from 1953-1968.
The vacuum tube amplifier
The vacuum tube (thermionic valve) amplifier was the breakthrough that enabled Adrian and Bronk to perform the first extracellular single axon recordings of motoneurons. They combined the vacuum tube amplifier and the capillary electrometer to measure the electric fields generated by single axons, but neither instrument was their invention.
Adrian's vacuum tube amplifier was based on that of Herbert Gasser (Gasser and Newcomer, 1921). During World War I, wireless telegraphy using vacuum tube amplifiers was developed for naval and battlefield communications. Electronic devices based on vacuum tube technology blossomed after the war and triggered a wave of interest in commercial and hobby electronics that still continues. To use them in the laboratory, neurophysiologists turned electronics hobbyists, and learned both principles of vacuum tube operation and the construction details required to make them perform as desired. Vacuum tubes were quirky, complicated devices.
The vacuum tube used by Adrian and Bronk had 3 signal ports, called cathode, grid, and plate. The voltage to be measured between two points on the nerve was applied between the grid of the first tube (dotted line) and ground. Electrons emitted from the cathode (the curved line at the tube bottom in the diagram) formed a beam that passed through the grid and were absorbed by the plate (straight line at the top). The plate was always kept positive compared to the cathode. The voltage difference between the cathode and grid regulated the plate-cathode current and so the effective resistance of the tube. When the grid became less positive, electrons from the cathode were repelled, and less current flowed between the plate and cathode, raising the effective resistance. If the grid to cathode voltage fell below a cutoff value, current stopped. Thus vacuum tubes could be used as electronic switches (in the binary circuits of digital computers in the 1940's and 1950's), but in an analog amplifier a constant bias voltage was applied to the grid (through resistor RG) to maintain a a positive grid to cathode voltage and a baseline plate-cathode current in the absence of any signal from the nerve. Both negative-going and positive-going voltage fluctuations at the grid could then produce changes in the plate current, resulting in amplified and inverted but otherwise similar output voltage changes at the output of the tube, taken from junction between plate and load resistor (RL). The useful part of this voltage at the next stage in the amplifier was the amplified fluctuation caused by changes in grid voltage, but it was superimposed on the constant voltage caused by the baseline plate-cathode current. To prevent that constant voltage from contaminating the input to the next amplifier stage, the output of each stage was coupled to the grid of the next by a large capacitor (CC) that effectively blocked constant current, but passed the more rapidly changing signal from the nerve. The amplification at each stage was determined by the intrinsic properties of the vacuum tube and the size of the load resistance. For the load resistor and vacuum tube used by Adrian and Bronk (100 kΩ and the Marconi DE 5B), the maximum achievable first stage gain should be about 15. By using 3 tubes in a sequence, the ideal total amplification was 3375. Adrian measured the amplification of their implementation at 1850, so achieved amplification between 12 and 13 at each stage (Adrian, 1926).
The voltage at the last amplifier stage was measured using a capillary electrometer. Before amplification was available, electrometers were chosen for sensitivity and frequency response, and extracellularly recorded single neuron action potentials were challenging on both counts, being both small (on the order of 0.1 mV) and brief (lasting < 1 ms). None of the electrometers commonly used to measure signals from whole nerve or muscle were adequate for single axons. The cathode ray tube oscilloscope, which would eventually replace them all, had already been introduced to neurophysiology by Gasser and Erlanger in 1922. However, the cathode ray tubes available at the time were too dim for photographic recording. To make a permanent record of the compound action potential, Gasser and Erlanger stimulated the nerve repeatedly to make a standing wave on the oscilloscope screen, and traced it by hand. Study of spontaneous asynchronous firing by single neurons using the oscilloscope would have to wait for improvements in the cathode ray tube.
Instead, Adrian and Bronk used the capillary electrometer, invented in the 1870s by Gabriel Lippmann and championed by Keith Lucas, Adrian's doctoral advisor. It consisted of a narrow tube of glass containing an interface between mercury and sulfuric acid. The interface rose and fell slightly in the capillary as the voltage difference between the two liquids changed. The capillary was illuminated from one side and movements of the interface were magnified with a microscope. A permanent record of voltage changes was obtained by exposing a photographic plate or paper as it moved across a narrow slit illuminated by the image of the interface. The capillary electrometer was not the most sensitive instrument available, but the liquid interface had a high contrast for photography and its low inertia gave it a moderately good frequency response.
In addition to the permanent photographic record, Adrian and Bronk also fed the amplified signals to a telephone headset or loudspeaker during the experiment to give immediate feedback about neuron firing in the form a a brief pop on each spike, beginning a neurophysiological custom that continues to this day.
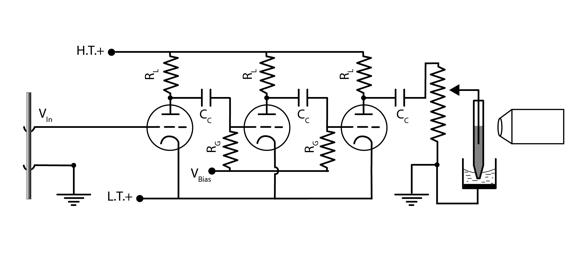
Schematic for Adrian three-stage amplifier and electrometer. Redrawn from Adrian, 1926a
Recording single axons required amplification, but it also required isolating the electrical signal from a single axon. Axons are crowded together in the nerve, and the electric field generated by an action potential in one is not readily distinguished from the collective field generated by all the others. The micro electrode, which would ultimately solve this problem by sampling the field in a tiny sub-volume of the tissue, had not yet been invented. Adrian and Bronk isolated the signal from a single axon the hard way, by cutting all the others so the nerve contained only one intact active axon. They divided the nerve successively into strands and cut them, eventually reducing it to a tiny intact strand containing only a few axons. When the strand was small enough so that only one active axon bridged the gap between the proximal and distal parts of the otherwise divided nerve, its signal could be taken downstream (distal) of the cut region using the conventional large "paintbrush" electrode normally used for recording compound action potentials. Of course the nerve could no longer function, and this created a problem for correlating motoneuron firing with muscle contraction (which was the entire point of the study).
In the first of the two Adrian and Bronk papers they described the firing of phrenic neurons controlling movements of the diaphragm during breathing. For this they used the phrenic branch of the third cranial nerve. Their dissection left it ineffective in breathing, but breathing continued because of the larger innervation by cranial nerves 4 and 5. In the second paper they studied discharge of motor axons to muscles of the leg during stimulus-evoked ipsilateral flexion and contralateral extension evoked by a foot pinch. For these they dissected small motor nerves close to their termination in the peroneus longus or tibialis anticus (for flexion) or quadriceps (extension) muscles. The intact innervation of the leg produced reflex movements whose strength and time course could be compared to action potentials of single axons in the dissected nerve. The assumption in both cases is that the small nerves they used for recordings were doing the same thing as the intact nerves whose function they could assess. This was be verified by recording from the intact nerve, or the dissected one proximal to the gap.